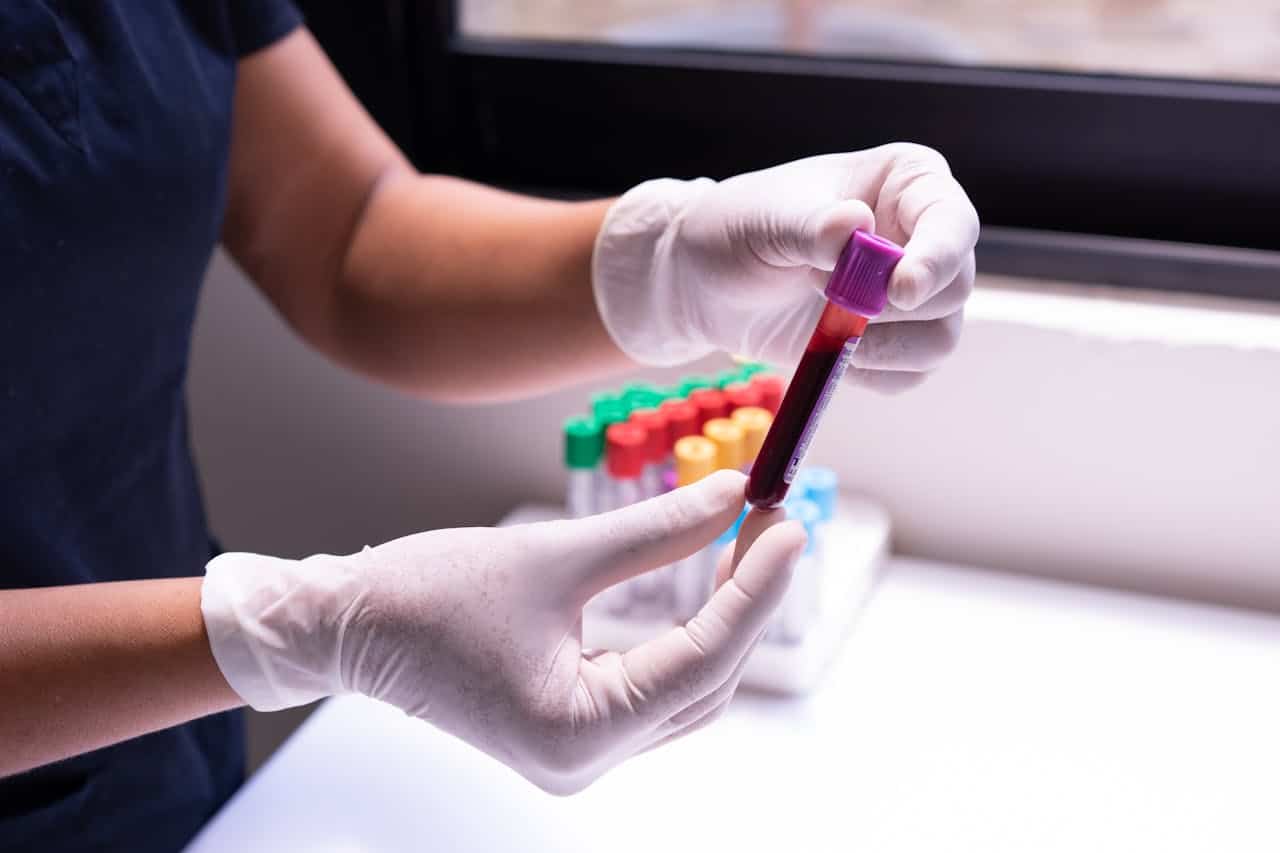
The U.S. Food and Drug Administration has authorized the use of the first blood test designed to help doctors detect Alzheimer’s disease earlier and more affordably. The decision marks a turning point in how the disease could be identified, especially for older adults showing early signs of memory decline.
Developed by Fujirebio Diagnostics, a Pennsylvania-based company, the test is intended for individuals aged 55 and older who are experiencing cognitive symptoms linked to Alzheimer’s. Unlike brain scans or spinal taps, which are costly and invasive, this new blood test offers a more accessible way to support a diagnosis.
The test works by measuring two specific proteins in the blood—pTau217 and beta-amyloid 1-42. When compared, the ratio of these proteins tends to indicate whether certain brain changes commonly associated with Alzheimer’s, known as amyloid plaques, may be present. While the test does not directly detect the plaques, the protein levels offer a strong clue about their existence.
A Step Toward Early and Easier Diagnosis
The FDA’s clearance was based on a study involving 499 adults showing signs of cognitive decline. Their blood samples were tested and the results were then matched with findings from brain scans or spinal fluid tests.
The data showed that over 91% of those who tested positive through the blood test also had amyloid plaques confirmed by more advanced procedures. Similarly, nearly 97% of those with negative test results showed no signs of plaques using those same benchmarks.
🧠🧪 FDA Approves First Blood Test to Detect Alzheimer’s Disease
— PiQ (@PiQSuite) May 16, 2025
Fujirebio’s test identifies amyloid plaques, enabling earlier, less invasive screening.
Positive results may guide patients toward advanced diagnostics and treatment. pic.twitter.com/lone2iXNwI
Despite its promise, health professionals caution that the blood test is not a standalone diagnostic tool. Doctors must still consider a patient’s overall health history, behavior changes, and results from other tests. The FDA emphasized that results from the blood test should only be used alongside additional clinical information.
FDA Commissioner Dr. Martin Makary noted the growing burden of Alzheimer’s, pointing out that about 10% of adults over age 65 in the U.S. currently have the disease. That number is expected to double by 2050. He expressed hope that tools like this blood test could help identify the condition earlier and potentially improve outcomes for patients.
Cautious Optimism From the Medical Community
Dr. Richard Isaacson, a preventive neurologist who helped launch one of the first Alzheimer’s prevention clinics in the U.S., praised the FDA’s decision.
He explained that this blood test, while not perfect, gives physicians a clearer view of whether memory loss could be linked to Alzheimer’s. He emphasized its value as a screening tool, especially when compared to expensive brain scans and more invasive spinal taps.
Still, Isaacson advised caution, noting that researchers and doctors must continue to study how to best interpret and apply the test results in real-world settings. He added that understanding how these results differ based on an individual’s risk factors remains a challenge.
Fujirebio Diagnostics CEO Monte Wiltse said the goal of the test is to identify Alzheimer’s as early as possible, allowing patients to begin treatment when it can be more effective. He also stressed that early and accurate detection could help guide future drug development, a growing priority as the global population ages.
Dr. Maria Carrillo from the Alzheimer’s Association called the FDA’s approval a significant development, saying it could make early diagnosis more available to more people. But she also pointed out that doctors must now figure out when the test should be used and for whom.
Dr. Howard Fillit of the Alzheimer’s Drug Discovery Foundation described the new test as a “game changer,” noting that a simple blood draw could soon help detect Alzheimer’s much like tests for cholesterol or diabetes. He said this development represents a broader shift in the field, as scientific advances begin to offer faster, easier, and more affordable ways to fight one of the world’s most common neurodegenerative diseases.